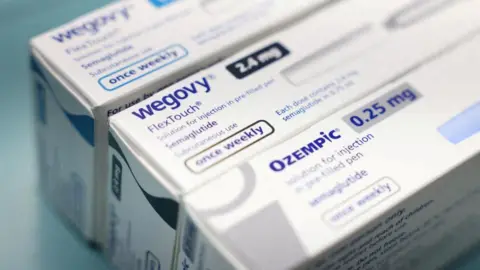
The US Food and Drug Administration (FDA) has approved a pill version of the weight-loss drug Wegovy, according to pharmaceutical giant Novo Nordisk.
This marks the first pill of its kind to receive FDA approval, a milestone for the weight-loss medication sector.
Wegovy's Danish makers, Novo Nordisk, described the once-daily pill as a convenient option compared to the injectable version, claiming it provides the same weight-loss benefits.
Previously, Wegovy was granted FDA approval for weight loss, while other treatments like Ozempic, which have similar effects, were primarily approved for managing Type 2 diabetes.
The Wegovy pill demonstrated an average weight loss of 16.6% during clinical trials, with a third of around 1,300 participants achieving a weight loss of 20% or more.
The FDA has yet to comment, but the pill is set to launch in the US in early January 2026.
Mike Doustdar, Novo Nordisk's chief executive, remarked: Patients will have a convenient, once-daily pill that can help them lose as much weight as the original Wegovy injection.
This new approval could bolster Novo Nordisk's sales following a challenging year, during which the company faced stiff competition from rivals like Eli Lilly. Following the announcement, Novo Nordisk's shares saw an almost 10% increase in after-hours trading in New York.